Pharmacovigilance
Welcome to our CPD-accredited online course on Pharmacovigilance for healthcare providers in Kenya, a comprehensive and free learning platform designed to enhance your professional skills and knowledge. Our Pharmacovigilance CPD-accredited course is offered by CRK clinical research organization and independently sponsored by Pfizer.
As a healthcare provider in Kenya, it’s crucial to stay updated with the latest developments in pharmacovigilance. This free CPD-Accredited course offers you the opportunity to do just that, providing an in-depth exploration of drug safety principles, adverse drug reactions (ADRs), and risk management strategies.
The CPD-accredited online course on Pharmacovigilance is tailored to the unique needs and challenges of the Kenyan healthcare system. It delves into the role of regulatory authorities in drug safety, providing you with a comprehensive understanding of the local and global pharmacovigilance landscape.
Our course is not just about theory; it’s about practical application. You’ll learn how to identify, assess, understand, and prevent adverse drug reactions, enhancing patient safety and improving treatment outcomes. The knowledge and skills you gain will directly apply to your daily practice, making you a more effective and informed healthcare provider.
Enroll in our CPD-accredited online course on Pharmacovigilance for healthcare providers in Kenya and take the next step in your professional journey.
What Will I Learn?
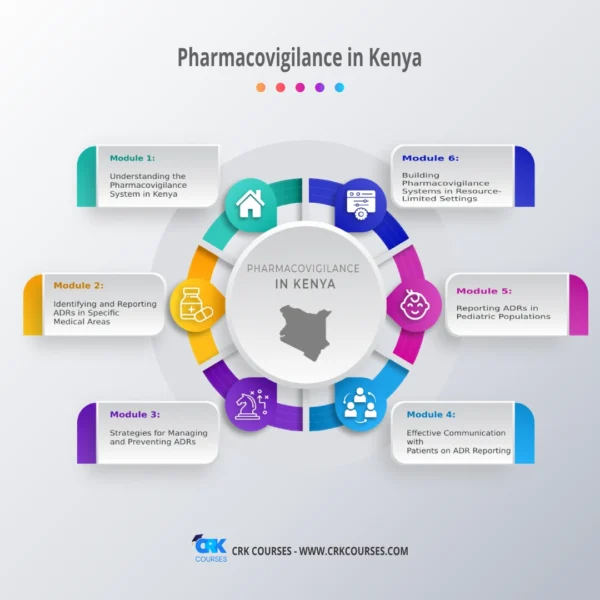
- Understanding the Pharmacovigilance System in Kenya
- Identifying and Reporting ADRs in Specific Medical Areas
- Strategies for Managing and Preventing ADRs
- Effective Communication with Patients on ADR Reporting
- Reporting ADRs in Pediatric Populations
- Building Pharmacovigilance Systems in Resource-Limited Settings
Course Content
Module 1: Understanding the Pharmacovigilance System in Kenya
Module 1 pre-test
Module 1 Overview
00:00Lesson 1: Introduction to Pharmacovigilance and Its Importance in Patient Safety
08:39Lesson 2: Pharmacovigilance Regulations and Reporting Requirements in Kenya
06:35Role-play : ADR Reporting Interaction at a Healthcare Facility
03:03Lesson 3: The process of reporting pharmacovigilance in Kenya
15:46Module 1 Assessment Test
Module 2: Identifying and Reporting ADRs in Specific Medical Areas
Module 3: Strategies for Managing and Preventing ADRs
Module 4: Effective Communication with Patients on ADR Reporting
Module 5: Reporting ADRs in Pediatric Populations
Module 6: Building Pharmacovigilance Systems in Resource-Limited Settings
About the instructor
7 Courses
7105 students
Great
best learning tool
Very insightful and educative
Excellent
The course is very educative and fits every healthcare provider.
Yes
good
Am particularly impressed by the simplistic models of knowledge delivery.
Pharmacovigilance is the basis of pharmacy practice. Having a good established pharmacovigilance will guarantee patients safety while using medications.
Such a deep knowledge you have provided really impact our profession even as we become patient centered. Thanks
EXCELLENT


